Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Metribuzin
Table of Contents
Guideline
The maximum acceptable concentration (MAC) for metribuzin in drinking water is 0.08 mg/L (80 µg/L).
Identity, Use and Sources in the Environment
Metribuzin (C8H14N4OS) is a triazine herbicide used for pre- and post-emergent weed control for a variety of agricultural crops. Between 100 000 and 500 000 kg are used annually in Canada.Footnote1 The solubility of metribuzin in water is 1.2 g/L at 20°C; its vapour pressure at 20°C is less than 1.3 x 10-3 Pa.Footnote2 The log octanol-water partition coefficient of metribuzin has been reported to be 1.70;Footnote3 therefore, it is not likely to bioaccumulate significantly.
Microbial degradation is the principal route of removal of metribuzin from the soil. Metribuzin is reported to be rapidly detoxified by deamination by the soil fungus Cunninghamella echinulata.Footnote 44 Metribuzin also moderately adsorbs to soil with high clay and/or organic matter content; adsorption decreases as soil pH increases.Footnote 2 The extent to which metribuzin leaches to groundwater is an inverse function of the organic matter content of soil.Footnote 2 Its half-life in soil ranges between 2.5 and four months.Footnote 5 Its half-life in pond water is approximately seven days.Footnote 6
Exposure
Metribuzin was detected in 26 of 1140 samples from municipal and private water supplies in Prince Edward Island (time period not reported), Nova Scotia (1986), Ontario (1979 to 1986), Manitoba (1986) and Alberta (1978 to 1986). Detection limits ranged from 0.01 to 1.0 µg/L. The maximum concentration of metribuzin, determined in a sample from a well in Ontario, was 300 µg/L.Footnote 7 Metribuzin was detected in six of 297 surface water samples (mean detected concentration 1.1 µg/L) from two Ontario river basins surveyed from 1981 to 1985; nearly 50 000 kg are used annually in these areas, based on application data from 1983 (detection limit 0.02 µg/L).Footnote 8

Download the entire report
(PDF format, 22 K, 3 pages)
Organization: Health Canada
Related Topics
The theoretical maximum daily intake of metribuzin in food is 0.12 mg/d, based on the residue tolerance limits established by the Food Directorate of the Department of National Health and Welfare.Footnote 9 No recent information on actual levels of metribuzin in foods was identified. Metribuzin was not detected in a survey of pesticide residues in the Canadian diet from 1976 to 1978 (detection limit 50 ppb).Footnote 10
Analytical Methods and Treatment Technology
Metribuzin in water may be determined by extraction with chloroform, separation by gas/liquid chromatography and quantification by electrolytic conductivity detector, nitrogen mode (detection limit 0.02 µg/L).Footnote 8 The analytical method proposed by the U.S. Environmental Protection Agency involves extracting with dichloromethane, drying, redissolving and concentrating the extract in acetone, separating by gas chromatography and measuring by thermionic bead detector (detection limit 0.46 µg/L).Footnote 11
Treatment by coagulation and sedimentation with alum (at an optimum dose of 200 mg/L) and an anionic polymer removes more than 50% of metribuzin from water supplies (initial concentration not reported); equivalent doses of ferric chloride were equally effective.Footnote 12 Two different granular activated carbon adsorption columns were found to be effective in the removal of metribuzin from water (96 and 100% for an initial concentration of 140 mg/L).Footnote 13
Health Effects
Rats administered 1 or 200 mg/kg bw of radioactively labelled metribuzin by stomach tube were reported to eliminate about 80% in the first day following administration, and 95% by the second day. Almost equal amounts were found in the urine and faeces. The major urinary metabolite was deaminometribuzin mercapturate, which accounted for 16 to 21% of the recovered radioactivity.Footnote 14 Metabolites identified in the tissues included the deaminated metabolite, the diketo metabolite and the deaminated diketo metabolite; the diketo metabolite is 2 to 3 times more toxic in rats than the parent compound, whereas the deaminated and deaminated diketo metabolites are of equivalent toxicity.Footnote 15
No reports on the effects of exposure of humans to metribuzin were identified in the literature. Metribuzin is considered to be relatively non-acutely toxic to mammals.Footnote 14
In a two-year feeding study in beagle dogs reviewed by the Food Directorate of the Department of National Health and Welfare, animals (four males and four females per group) ingested diets containing 0, 25, 100 or 1500 ppm metribuzin (99.5% pure). The authors determined that these doses were equivalent to 0, 0.83, 3.5 or 55.5 mg/kg bw per day, respectively, based on consumption and weight data. Food consumption and body weight gain were reduced in the highest dose group (55.5 mg/kg bw per day); thyroid weight in males and females and liver, spleen and kidney weights in males were increased relative to body weights. At 3.5 mg/kg bw per day, there was an increase in the incidence of necrobiosis of the liver (4/8 in treated animals, 1/8 in controls). The significance of this increase is uncertain, because of the lack of historical control data on the occurrence of necrobiosis in dogs. At 3.5 mg/kg bw per day, an increase in the incidence and severity of mucopolysaccharide droplets in the lobular periphery of the liver was also noted. No treatment-related effects were observed at 0.83 mg/kg bw per day, which the authors considered to be the no-observed-adverse-effect level (NOAEL).Footnote 15
In a two-year carcinogenicity study in albino CD-1 mice, animals (50 per sex per dose) were administered diets containing 200, 800 or 3200 ppm. No significant increase in any specific type of tumours was observed at any dose level.Footnote 16
Metribuzin was not mutagenic in several bacterial assays or microbial point mutation assays. The results of a dominant lethal test in mice were negative, and metribuzin did not induce chromosomal aberrations in hamster spermatogonia.Footnote 11
In a teratology study in which pregnant rabbits were administered doses of 15, 45 or 135 mg/kg bw per day on days 6 through 18 of gestation (16 to 17 animals per dose), toxic effects such as decreased body weight gain were observed in the dams at the highest dose (135 mg/kg bw). There were no effects at any dose level in the foetuses, based on gross, soft tissue and skeletal examinations.Footnote 17 No toxic effects in the mothers, embryotoxicity or teratogenic effects were observed in FB30 rats administered doses of 5, 15, 50 or 100 mg/kg bw per day of metribuzin via stomach tube on days 6 through 15 of gestation.Footnote 18
There were no adverse effects in a three-generation reproductive study in FB30 rats administered doses of 35, 100 or 300 ppm in the feed during mating, gestation and lactation, based on evaluation of fertility, lactation performance and pup development.Footnote 19
Rationale
The acceptable daily intake (ADI) for metribuzin has been derived by the Food Directorate of the Department of National Health and Welfare as follows:
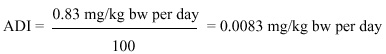
where:
- 0.83 mg/kg bw per day is the NOAEL in the two-year study in dogsFootnote 1515
- 100 is the uncertainty factor.
The maximum acceptable concentration (MAC) for metribuzin in drinking water is derived from the ADI as follows:

where:
- 0.0083 mg/kg bw per day is the ADI derived by the Food Directorate
- 70 kg is the average body weight of an adult
- 0.20 is the proportion of daily intake of metribuzin allocated to drinking water (available data are insufficient to estimate this value)
- 1.5 L/d is the average daily consumption of drinking water for an adult.
References
Footnotes
Report a problem or mistake on this page
- Date modified: