R&D Blueprint
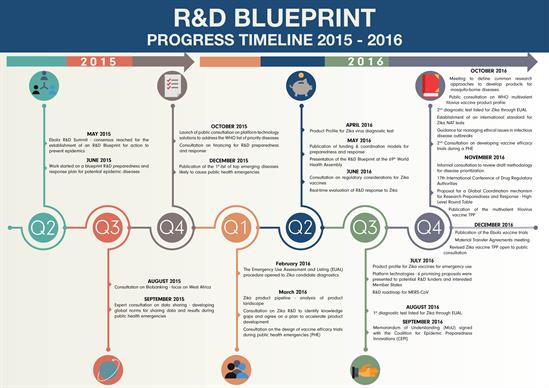
The R&D Blueprint is a global strategy and preparedness plan that allows the rapid activation of research and development activities during epidemics. Its aim is to fast-track the availability of effective tests, vaccines and medicines that can be used to save lives and avert large scale crises. With WHO as convener, the broad global coalition of experts who have contributed to the Blueprint come from medical, scientific and regulatory backgrounds. WHO Member States welcomed the development of the Blueprint at the World Health Assembly in May 2016.
Key actions by disease
Publications

These landscape documents have been prepared by the World Health Organization (WHO) for information purposes only concerning the 2019-2020 global of the...

29 April 2020
COVID-19 Social Science working group
The COVID-19 social science research agenda aims to (1) generate high quality social science evidence for achieving the goals of national strategic public...

This large, international, randomized controlled clinical trial is designed to enable an expeditious, agile and concurrent evaluation of the benefits and...

10 April 2020
WHO R&D Blueprint COVID-19: Informal consultation on the potential inclusion of Favipiravir in a clinical...
There has been some suggestions for the inclusion of Favipiravir in the Solidarity trial.

Currently, there are no licensed vaccines for the prevention of COVID 19. While efforts continue to develop effective vaccines, it is pertinent to examine...